Abstract
Background: serum protein electrophoresis (SPEP) and B-cell receptor next generation gene sequencing (NGS) are methodologies used to monitor the amount of a monoclonal protein in multiple myeloma (MM) patients and to evaluate minimal residual disease (MRD), respectively. Mass spectrometry (MS) is a methodology currently being explored to provide a serum/plasma-based quantitative assessment of M-protein, which could also serve as a surrogate of MRD. Here we present the analytical performance of SPEP and MRD compared to the MALDI-TOF MS-based assay EXENT.
Methods: SPEP, NGS (Clonoseq v 2.0, Adaptive Biotechnologies), and MS (EXENT®, The Binding Site) assays were performed to assess M-protein in 65 serum samples from 22 MM patients who participated in a phase 3 clinical trial (CASTOR, NCT02136134), in which patients received Daratumumab, Bortezomib and Dexamethasone (DVd) or Bortezomib and Dexamethasone (Vd). Irrespective of treatment, serum samples collected at three time points were selected from each subject, including 1) baseline prior to treatment, 2) available sample at or most approximately prior to the first confirmed complete response (CR) according to IMWG criteria with SPEP equaled 0, and 3) last available sample prior to disease progression (PD) with SPEP equaled 0. M-protein concentration measured by EXENT was compared to that measured by SPEP. Data was analyzed by using either all obtained values (n=62) or only non-zero values (n=18) by EXENT or SPEP. NGS-based MRD results (n=26) used for the comparison were those sampled within 60 days of the samples used for the EXENT analysis.
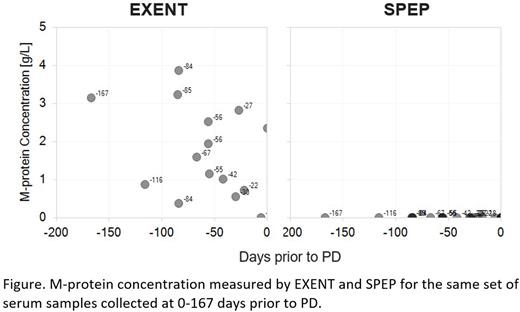
Results: M-protein concentration by EXENT significantly correlated with SPEP measurements by using all obtained values (r=0.986, p<0.0001). The correlation was also significant when non-zero values were used in the analysis (r=0.969, P<0.0001). The lower limit of quantification (LLOQ) of EXENT was 0.015 g/L, while that of SPEP was approximately 1 g/L. With the better sensitivity, EXENT was able to detect M-protein up to 167 days prior to PD when SPEP detected none (figure). MS-based assessments were concordant with NGS-based MRD results. A 76.9% (20 of 26) agreement was observed when comparing EXENT with MRD at a sensitivity of 10-5. EXENT purified samples were reflexed to analysis by the more sensitive MS-based methodology LC-MS. The agreement between LC-MS and MRD at a sensitivity of 10-6 was 92.3% (24 of 26). In addition, we observed several useful features unique to MS-based assays. These include distinguishing daratumumab from the endogenous M-protein, detecting isotype switching, and the ability of detecting light chain glycosylation.
Conclusion: EXENT significantly correlated with SPEP while EXENT demonstrated a higher sensitivity in quantification of M-protein in serum than SPEP. A high degree of agreement was observed between EXENT/LC-MS and NGS-based MRD. With its non-invasive nature and early detection of relapse, EXENT can be used as a complementary assay in MM patient management to guide bone marrow based MRD assessment.
Disclosures
Li:Janssen R&D, Johnson and Johnson: Current Employment. Barnidge:The Binding Site Group Ltd: Current Employment. Krevvata:Janssen R& D, Johnson and Johnson: Current Employment. Perkins:The Binding Site Group Ltd: Current Employment. Verona:Janssen R&D, Johnson and Johnson: Current Employment. Zudaire:Janssen R&D, Johnson and Johnson: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal